Overview
I am neither an alarmist or a denier. I am a realist. This is an attempt to realistically see what effect increased methane in the atmosphere is going to have. There are complex feedbacks in this process both positive and negative. Positive feedbacks are those that re-enforce a process, negative feedbacks work against the process.
The primary sink for methane is its destruction in the atmosphere by hydroxyl radicals this would be considered a negative feedback. Before it gets into the atmosphere methane is also destroyed by a collection of bacteria called methanotrophs, another negative feedback. However, there are many positive feedbacks that will encourage more methane to be released.
However, with reduced Hydroxyls what will remove the methane from the atmosphere? It pays to look at the various forces that create Hydroxyls and a powerful one is lightning.
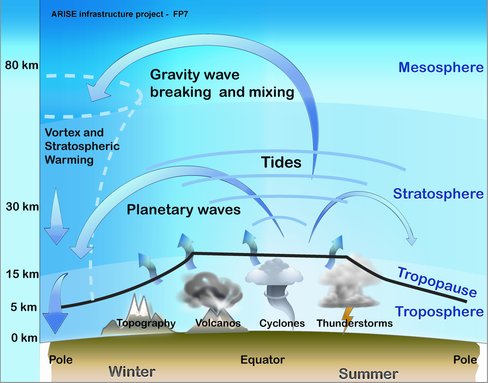
The following is an overview of atmospheric movements from this page.
The primary sink for methane is its destruction in the atmosphere by hydroxyl radicals this would be considered a negative feedback. Before it gets into the atmosphere methane is also destroyed by a collection of bacteria called methanotrophs, another negative feedback. However, there are many positive feedbacks that will encourage more methane to be released.
Hydroxyl
Hydroxyl breaks down methane in the atmosphere. A 2009 NASA article states a 26% decrease in hydroxyl, primarily due to increasing methane levels.
In 2014, researchers reported their discovery of a "hole" or absence of hydroxyl throughout the entire depth of the troposphere across a large region of the tropical West Pacific. They suggested that this hole is permitting large quantities of ozone-degrading chemicals to reach the stratosphere, and that this may be significantly reinforcing ozone depletion in the polar regions with potential consequences for the climate of the Earth.
There is a lack of hydroxyl in the Arctic atmosphere. meaning the methane will lift to the stratosphere. This lack of hydroxyl is related to the angle of the sun at the poles.
However, with reduced Hydroxyls what will remove the methane from the atmosphere? It pays to look at the various forces that create Hydroxyls and a powerful one is lightning.
Here, we show that the reaction of electronically excited nitrogen dioxide with water can be an important source of tropospheric hydroxyl radicals. Using measured rate data, along with available solar flux and atmospheric mixing ratios, we demonstrate that the tropospheric hydroxyl contribution from this source can be a substantial fraction (50%) of that from the traditional O(1D) + H2O reaction in the boundary-layer region for high solar zenith angles. (link)
For every one degree Celsius of long-term warming there will be a near 10 percent increase in lightning activity. "During El Nino years, which occur in the Pacific Ocean or Basin, Southeast Asia gets warmer and drier. There are fewer thunderstorms, but we found fifty percent more lightning activity," says Prof. Price. Typically, he says,we would expect drier conditions to produce less lightning. However, researchers also found that while there were fewer thunderstorms, the ones that did occur were more intense. (link)Here is an example from the recent bush fires in Australia. The yellow dots were lightning strikes from an incoming front that really brought no significant rain. The blue dots were active fires.
Brewer-Dobson circulation
(link)
However, the polar vortex is weakening. Assuming that it is weakening due to a warming arctic, then it follows that continual warming will result in continued weakening of the vortex. This will draw down less air from the stratosphere. Resulting in the methane remaining in the stratosphere.
Compared to June 2013, mean methane levels at higher altitudes are now well over 10 ppb higher at higher altitudes while there has been only little change closer to the ground. (link)
Stratospheric Methane
There seems to be conflicting evidence for the lifetime of methane in the stratosphere. Once account states that even if it is not destroyed in the troposphere, methane can usually only last 12 years before it is eventually destroyed in the stratosphere. Destruction in the stratosphere occurs the same way that it does in the troposphere: methane is oxidized to produce carbon dioxide and water vapor. (link)Another has it as the lifetime of methane against OH oxidation was found to be about 168 days at 25 km and 26 days at 45 km (link) Steve Rieck
So it appears methane is oxidized faster at higher altitudes.
Aerobic methanotrophs and are usually found in well-drained soils or similar environments where they have a good supply of both oxygen and methane. The uptake of methane by these micobes constitutes only about five percent of the total methane sink of between 500 and 600 million tonnes per year. However, the methanotrophs are more important than this figure might indicate, as they also consume a great deal of methane before it is released to the atmosphere.
There is an interesting correlation between the Arctic and the 2010 Deepwater Horizon blowout as most of the gas injected into the gulf was methane.
"For these bacteria to work efficiently, they need unlimited access to nutrients like inorganic nitrogen and trace metals, but they also need elevated methane levels to persist long enough to support high rates of consumption," Joye said. "The bacteria in the Gulf were probably able to consume about half of the methane released, but we hypothesize that an absence of essential nutrients and the dispersal of gas throughout the water column prevented complete consumption of the discharged methane." (link)
In the case of the Arctic the gas will be available, but the question is whether the correct nutrients will be. It is not an area with high levels of agricultural runoff. However, the Arctic ocean contains high levels of phosphate and because of the riverine flows, it contains a good deal of Dissolved Nitrogen.(link) To what degree these nutrients will support a massive increase in the needed aerobic methanotrophs will probably only be known after the fact.
Anaerobic methanotrophs are typically found in the sediments around methane hydrates. They are interesting in that one in particular, Candidatus Methylomirabilis oxyfera has the ability to generate oxygen in the absence of light.
Hydroxyl increase and decrease
Methanotrophs
Significant amounts of methane are also oxidised by microorganisms (called methanotrophs) which use the methane as a source of carbon and energy. Methanotrophs can be aerobic or anaerobic.Aerobic methanotrophs and are usually found in well-drained soils or similar environments where they have a good supply of both oxygen and methane. The uptake of methane by these micobes constitutes only about five percent of the total methane sink of between 500 and 600 million tonnes per year. However, the methanotrophs are more important than this figure might indicate, as they also consume a great deal of methane before it is released to the atmosphere.
There is an interesting correlation between the Arctic and the 2010 Deepwater Horizon blowout as most of the gas injected into the gulf was methane.
"For these bacteria to work efficiently, they need unlimited access to nutrients like inorganic nitrogen and trace metals, but they also need elevated methane levels to persist long enough to support high rates of consumption," Joye said. "The bacteria in the Gulf were probably able to consume about half of the methane released, but we hypothesize that an absence of essential nutrients and the dispersal of gas throughout the water column prevented complete consumption of the discharged methane." (link)
In the case of the Arctic the gas will be available, but the question is whether the correct nutrients will be. It is not an area with high levels of agricultural runoff. However, the Arctic ocean contains high levels of phosphate and because of the riverine flows, it contains a good deal of Dissolved Nitrogen.(link) To what degree these nutrients will support a massive increase in the needed aerobic methanotrophs will probably only be known after the fact.
Anaerobic methanotrophs are typically found in the sediments around methane hydrates. They are interesting in that one in particular, Candidatus Methylomirabilis oxyfera has the ability to generate oxygen in the absence of light.
Feedbacks (negative and positive)
If we end up with methane rapidly moving into the stratosphere what effects can be expected?
The reduction of sea ice causes the surface reflectivity to strongly decrease in the Arctic, which leads to decreases in shortwave radiation in the atmosphere and, thus, lower photo-dissociation rates of tropospheric gases. Photo-dissociation of the ozone molecule is the major process that leads to the production of OH (hydroxyl radical), the main oxidizing (i.e., cleansing) gas species in the troposphere. Therefore, we expect that a decrease in photo-dissociation rates would lead to a decrease in OH concentrations and a weakening of the oxidizing capacity of the Arctic troposphere.
(link)
A lot depends on the Hydroxyl radicals in the stratosphere. At this point it does not appear that there is a significant decline in the stratospheric hydroxyl radicals. (link). That might change though with the forementioned effects in the troposphere. However, the increased emissions of methane from the hydrates would also lead to a population explosion of methanotrophs. The water depth in the Arctic sea is shallow, so Aerobic Methanotrophs would not be able to consume sufficient methane before it hit the surface. Anaerobic Methanotrophs however, are in the anoxic sediments of the sea beds, as the sediments thawed these bacteria would thrive. Given the quantities of methane, the anaerobic type would also be overwhelmed.
The conclusion then would be that any sizable release of methane from the hydrates would have a violent warming effect for the Arctic. There would be positive feedbacks that would continue to warm the Arctic rapidly creating more methane. However, it appears that methane may last in the atmosphere for a short period. A weakening of the polar vortex would cause the methane to be held closer to the North Pole. That same methane would however, be reducing the hydroxyls in the atmosphere, which are not abundant at the poles to begin with.
A negative feedback from such a rapid warming would result from its the effect on the Greenland ice sheet. Massive quantities of fresh water would be forced into the Arctic from Greenland. In addition the Thermohaline circulation would be slowed down. This dealt with in more detail here. The fresh water from Greenland would have a high oxygen content which would encourage the growth of Aerobic Methanotrophs.
As shown elsewhere, the effects of increased methane might well be felt more in the Northern Hemisphere than in the South. From the Nuclear Pollution post;
Another variable in the process is that air circulation appears to be slowing down. (link) From the article "By the end of the century, more than half of the world’s population will be exposed to increasingly stagnant atmospheric conditions, with the tropics and subtropics bearing the brunt of the poor air quality." Stagnant air at the tropics would mean more opportunity for the nuclear pollutants to be cleared from the atmosphere by precipitation. So whatever the N. Hemisphere does to itself does not immediately translate into the same effect in the south.
A negative feedback from such a rapid warming would result from its the effect on the Greenland ice sheet. Massive quantities of fresh water would be forced into the Arctic from Greenland. In addition the Thermohaline circulation would be slowed down. This dealt with in more detail here. The fresh water from Greenland would have a high oxygen content which would encourage the growth of Aerobic Methanotrophs.
As shown elsewhere, the effects of increased methane might well be felt more in the Northern Hemisphere than in the South. From the Nuclear Pollution post;
Another variable in the process is that air circulation appears to be slowing down. (link) From the article "By the end of the century, more than half of the world’s population will be exposed to increasingly stagnant atmospheric conditions, with the tropics and subtropics bearing the brunt of the poor air quality." Stagnant air at the tropics would mean more opportunity for the nuclear pollutants to be cleared from the atmosphere by precipitation. So whatever the N. Hemisphere does to itself does not immediately translate into the same effect in the south.
Hydroxyl increase and decrease
This view should be balanced out by a decrease in the Hydroxyl radicals in the stratosphere due its combination with the increased methane. Also as stated previously there is less Hydroxyl radicals in the Arctic atmosphere. So the Arctic will warm faster than other areas, which is currently happening.
This in turn needs to be considered against the production of hydroxyl at oxic - anoxic boundaries of water and soil.
Hydroxyl radical (•OH) is a highly reactive and unselective oxidant in atmospheric and aquatic systems. Current understanding limits the role of DOM-produced •OH as an oxidant in carbon cycling mainly to sunlit environments where •OH is produced photochemically, but a recent laboratory study proposed a sunlight-independent pathway in which •OH forms during oxidation of reduced aquatic dissolved organic matter (DOM) and iron. Here we demonstrate this non-photochemical pathway for •OH formation in natural aquatic environments. Across a gradient from dry upland to wet lowland habitats, •OH formation rates increase with increasing concentrations of DOM and reduced iron, with highest •OH formation predicted at oxic-anoxic boundaries in soil and surface waters. Comparison of measured vs expected electron release from reduced moieties suggests that both DOM and iron contribute to •OH formation. At landscape scales, abiotic DOM oxidation by this dark •OH pathway may be as important to carbon cycling as bacterial oxidation of DOM in arctic surface waters. (link)
As can be demonstrated here, the oceans are quickly moving into oxygen depletion. So new hydroxyls are being created while others are being consumed.
The other factor mentioned above is the increase in lightning that also generates hydroxyls.
Conclusion
In no way am I saying that the methane releases will be mitigated by these effects. This involves highly complex reactions between chaotic systems that no model can predict. We can however, expect there to be dramatic warming in the Arctic with serious implications for the rest of the planet. Given the mentioned negative feedbacks, how long that warming effect lasts is uncertain.
In my opinion it is too simplistic to draw a conclusion that the end result will be an environment incompatible with human life at all. However, I doubt that civilization as we have experienced it could continue. What is certain though is that humanity has developed in a stable environment. It will be unprepared for this.
Once the effects of the methane releases are being felt by the general public, panic will result. One can then assume that engineering projects that create negative feedbacks will be more acceptable.
In my opinion it is too simplistic to draw a conclusion that the end result will be an environment incompatible with human life at all. However, I doubt that civilization as we have experienced it could continue. What is certain though is that humanity has developed in a stable environment. It will be unprepared for this.
Once the effects of the methane releases are being felt by the general public, panic will result. One can then assume that engineering projects that create negative feedbacks will be more acceptable.


No comments:
Post a Comment